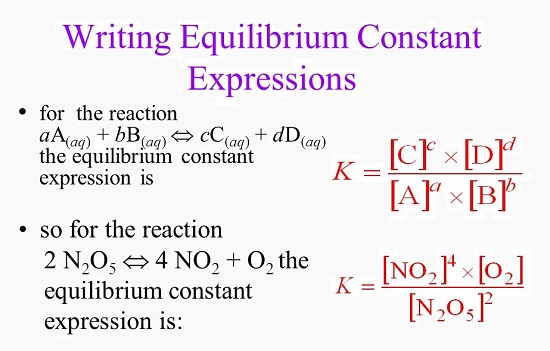
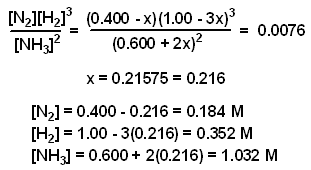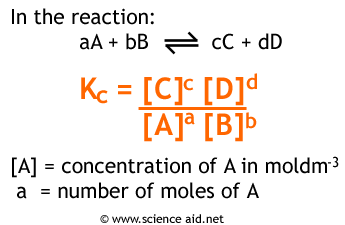Calculate the equation constant for the reaction: H2 (g) + CO2 (g) H2O (g) + CO at 1395 K, if the equilibrium constants at 1395 K for the following are 2H2O (g)
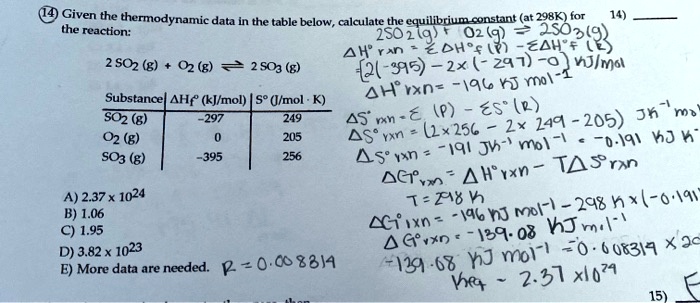
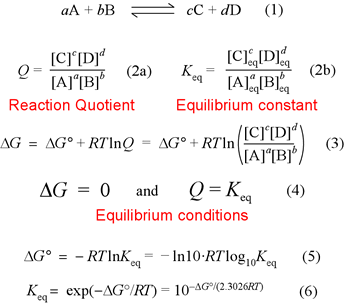
SOLVED: Given - the thermodynamic data in the table below, calculate the equilibrium constant (at 298K) for the reaction: 250 2 02 2502 9 3 Z4H" = 8 4h" in Dh"f SO2 (

Is the equilibrium constant calculation only applicable to homogeneous reactions? - Chemistry Stack Exchange

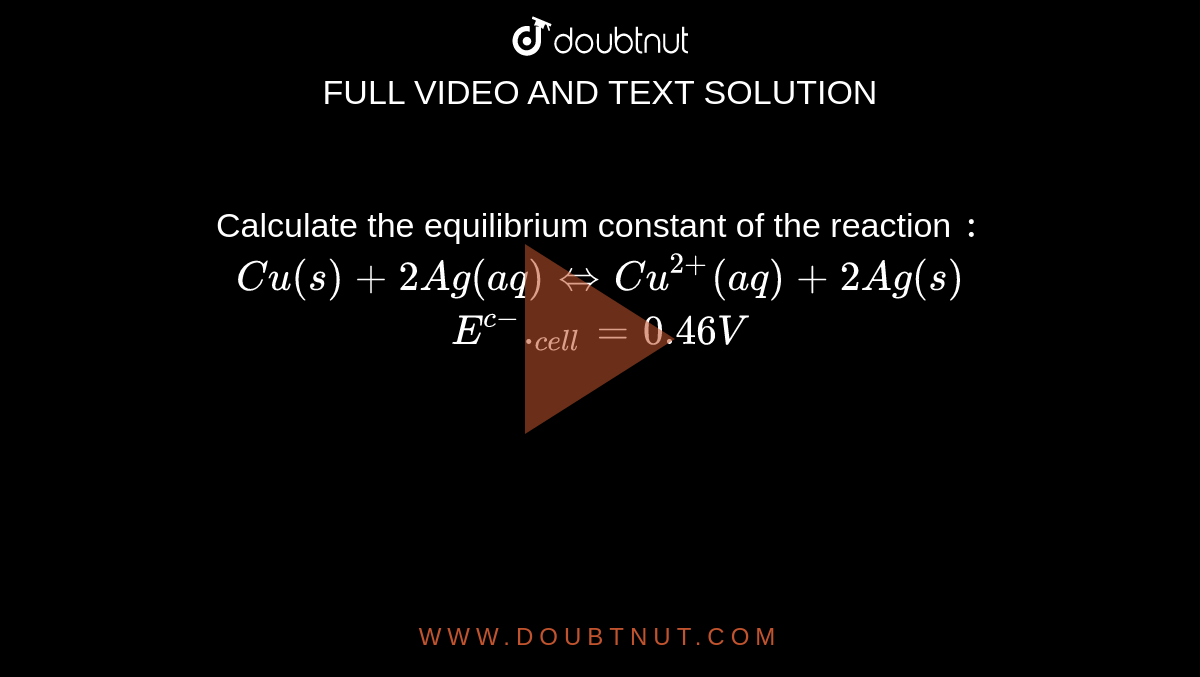
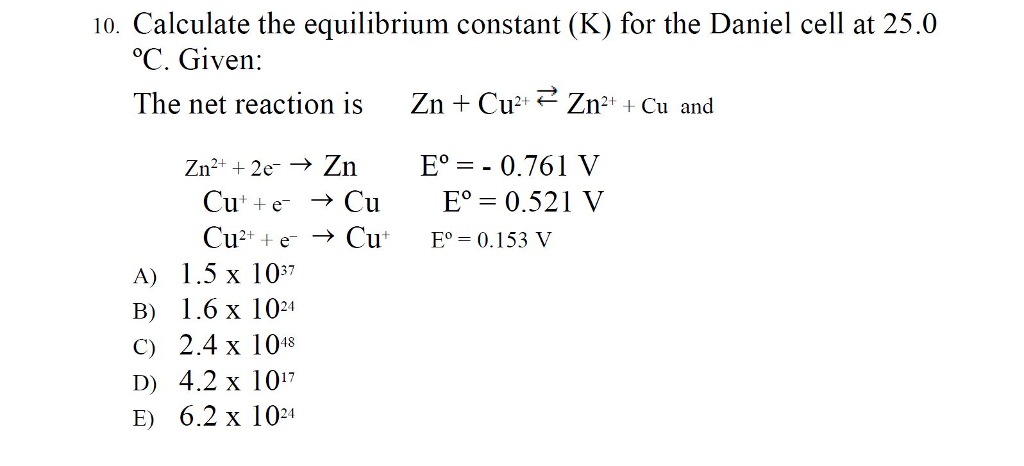
Calculate the equilibrium constant for the reaction, at 25^oC Cu(s) + 2Ag^ + (aq) → Cu^+2 (aq) + 2Ag (s)at 25^oC , E^o cell = 0.47 V, R = 8.134 JK^-1 F = 96500 C is

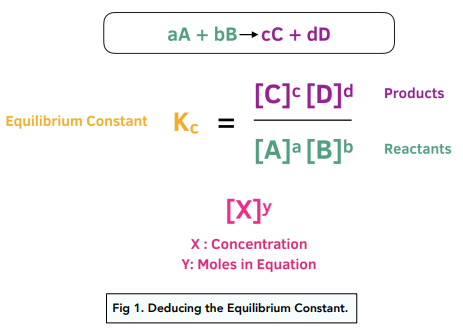
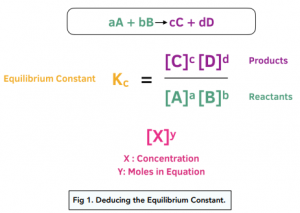
7.2 The Position of Equilibrium.. Assessment Statements Deduce the equilibrium constant expression (K c ) from the equation for a homogeneous reaction. - ppt download

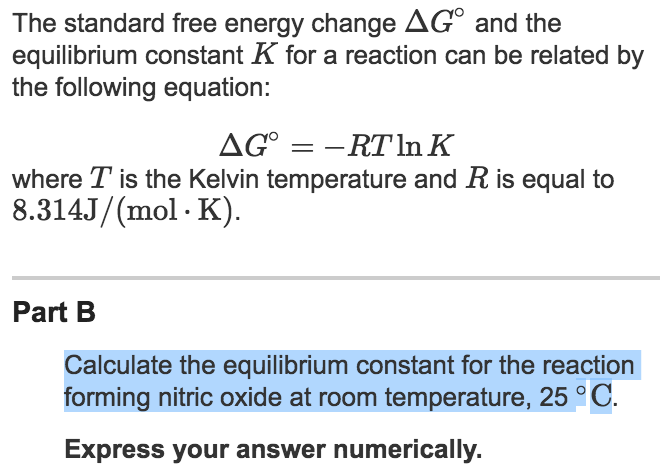
OneClass: Calculate the equilibrium constant, K, for the following reaction at 25 degree C. Fe^3 + (a...