Given H2(g) + Br2(g)→ 2HBr(g),Δ H^01 & standard enthalpy of condensation of bromine is Δ H^02 , standard enthalpy of formation of HBr at 25^0C is
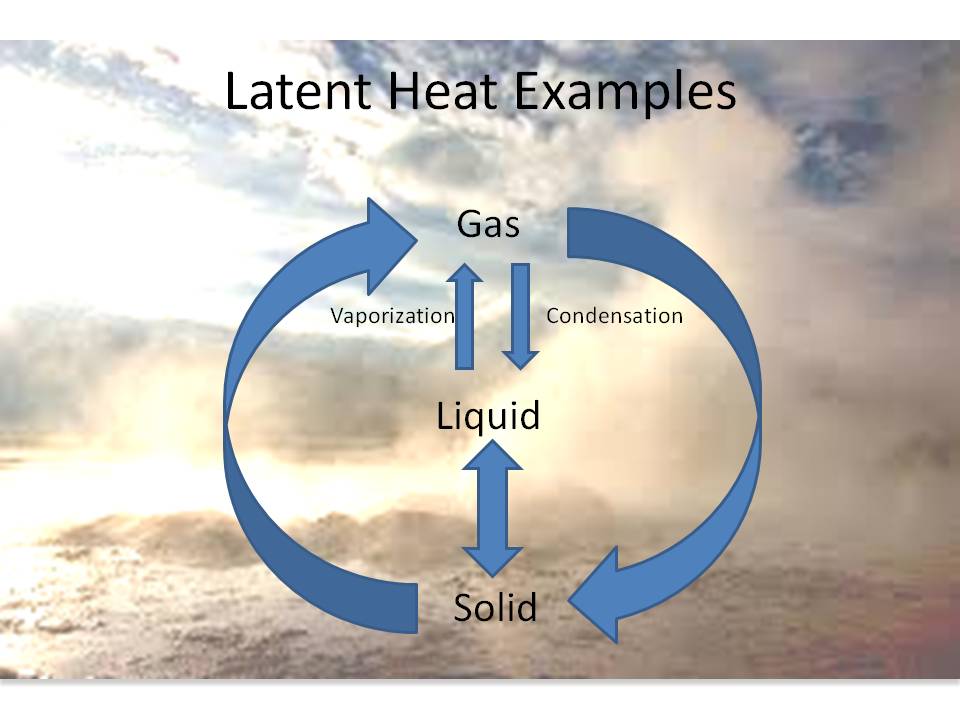
What's the difference between latent and sensible heat on the BPI exam? Take your free BPI practice exam here. - Online Energy Auditor Certification Training Course

Do Now 2NaHCO kJ Na 2 CO 3 + H 2 O + CO 2 Is this an endothermic or exothermic reaction? Calculate the amount of heat transferred when 36 g of. - ppt download
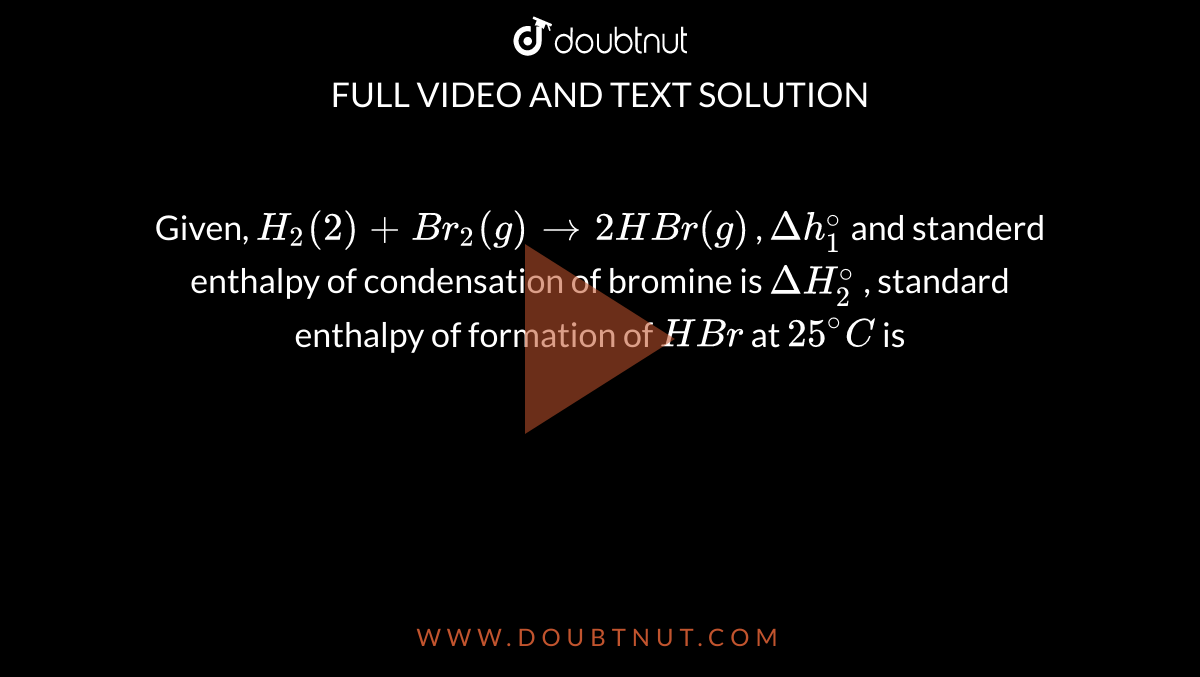
Given, H(2)(2)+Br(2)(g)rarr2HBr(g) , Deltah(1)^(@) and standerd enthalpy of condensation of bromine is DeltaH(2)^(@) , standard enthalpy of formation of HBr at 25^(@)C is

Assuming the enthalpy of vaporization is 10 kJ/mol and the gas's molar volume at the onset of condensation is 1 L/mol, what is the enthalpy change for the C->B process is (in

Given, `H_(2)(g)+Br_(2)(g)to2HBr(g),DeltaH_(1)^(@)` and standard enthalpy of condensation of - YouTube